Researchers from the National University of Quilmes in collaboration with colleagues from the Technical University of Dresden in Germany propose a new paradigm about the process of insulin biosynthesis.
Researchers from the Structural Biology and Biotechnology Group of the National University of Quilmes linked to the Multidisciplinary Institute of Cellular Biology (IMBICE‒CONICET–UNLP) in collaboration with the laboratory of Dr. Michele Solimena of the Paul Langerhans Institute of the Faculty of Medicine of the Technische Universität Dresden, Germany, published new advances in the study of diabetes from the perspective of modern structural biology. The article was recently published in the renowned journal Protein Science.
ICA512 and phogrin are membrane proteins expressed in pancreatic beta cells. In these cells, both proteins are directly involved in the biosynthesis and turnover of insulin secretion granules (SG), and their dysfunction ultimately leads to diabetes development.
In recent years, the groups from Argentina and Germany have focused on the study of the N-terminal domain of ICA512 called RESP18HD, which has sequence homology with another SG protein called Regulated endocrine specific protein 18 (Resp18). Increased glucose concentration stimulates the expression of these proteins which promote the biogenesis of new insulin SG in beta cells.
“A few years ago we showed that RESP18HD inhibits the formation of insulin amyloid-like fibres in vitro and also showed that together—RESP18HD and insulin—they form amorphous particles that precipitate”, explains Dr Pamela Toledo, postdoctoral fellow at CONICET and first author of the work. “In this work—she continues—we expand the studies of the RESP18HD-insulin interaction with the precursor protein, proinsulin. The formation of insulin SG is the last event in the pathway. For this, numerous processes must first occur under different physicochemical conditions and that is where proinsulin is involved”.
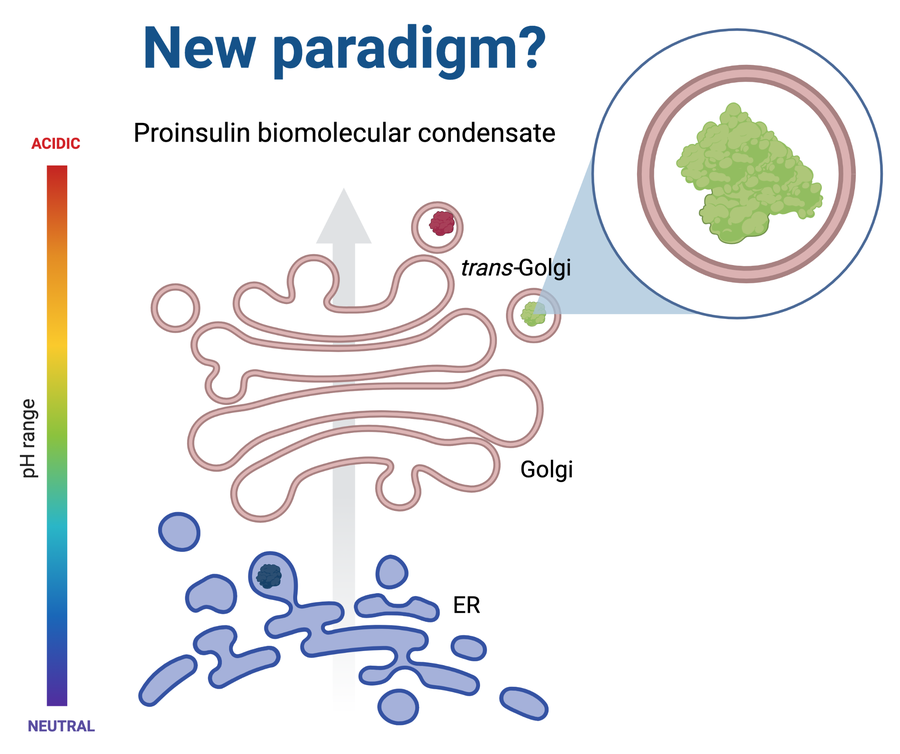
“This work is part of a biophysical and biochemical process that is in a stage of intense research throughout the world, the biomolecular condensates. We can imagine a biocondensate like having a drop of a liquid inside another liquid that coexists in harmony but does not mix and these drops are made, in this case, of one or more proteins but also of genetic material such as RNA and proteins. What we were able to observe is that proinsulin by itself is capable of forming these biocondensates at a pH similar to that found in the secretory pathway”, adds Dr Diego S. Vazquez, researcher from CONICET and co-author of the study. “This ability of proinsulin surely plays a very important role in the correct folding of the protein, which ultimately allows insulin stores to form correctly and be available when the body needs it,” adds the researcher.
“There are more and more examples of cellular processes where biomolecular condensates intervene in a normal way, but it is also a phenomenon associated with numerous neurodegenerative diseases such as Alzheimer disease and also it was observed in the formation and replication of different viruses, which transforms the subject is a hot topic“ add the researchers.
Researchers: Pamela L. Toledo. Postdoctoral fellow CONICET Diego S. Vazquez. Assistant Researcher CIC-CONICET Alejo R. Gianotti. Postdoctoral fellow CONICET Milagros B. Abate. Undergraduate student UNQ Juha M. Torkko. UT, Dresde, Germany Carolin Wegbrod. UT, Dresde, Germany Michele Solimena. UT, Dresde, Germany Mario R. Ermácora. Principal Researcher CIC-CONICET Link to the article: Toledo PL, Vazquez DS, Gianotti AR, Abate MB, Wegbrod C, Torkko JM, Solimena M, Ermácora MR. Condensation of the β-cell secretory granule luminal cargoes pro/insulin and ICA512 RESP18 homology domain. Protein Sci. 2023 May 9. DOI: 10.1002/pro.4649.